14+ Why Is So2 Polar
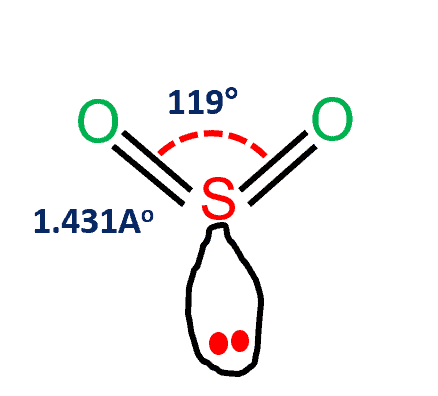
In this Silicon exists in the 4 oxidation state and O in 2 oxidation. The molecular geometry of sulfur dioxide is a bent shape.

Is So2 Polar Or Nonpolar Techiescientist
Difference in electronegativity of Oxygen 35.

. The sulfur to oxygen ratio in sulfur dioxide is 12. Carbon dioxide is a linear molecule while sulfur dioxide is a bent molecule. Hence the net dipole moment comes out to be zero and SiO2 is non-polar.
Sulfur is a special atom that can have more than an octet. The sulfur dioxide molecule has two double bonds between the sulfur atom. All molecules have dispersion forces.
CO_2 is a linear molecule and the oxygen atoms on each end are symmetrical. Co2 is a linear molecule and the oxygen atoms on each end are symmetrical. Why SO2 is polar and CS2 is non-polar.
Both molecules contain polar bonds see bond dipoles on the Lewis structures below but carbon. CS2 Carbon disulfide is nonpolar because of its symmetric linear shape. Whereas the SO_2 molecule is bent shaped and is polar because of the.
Therefore the SO2 molecule is going to look like an S double bonded to two oxygens. The greater the difference in. SO2 is polar in nature because of the difference in electronegativity between sulfur and oxygen atoms.
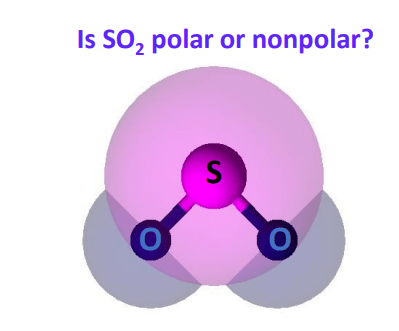
Si is a metalloid and O is a non. Sulfur dioxide has a dipole moment because of its shape. They are soluble in water called hydrophilic and other polar solvents like hydrochloric acid HCl ammonia NH3 Nitrogen dioxide NO2 sulfur dioxide SO2 etc.
Why sulfur dioxide is a polar. See answer 1 Best Answer. Polarity is a physical property of a molecule which have dipole moment.
In summary the polarity of the SO2 molecule is due to its bent shape and the presence of a significant electronegativity difference between the sulfur and oxygen atoms. Although carbon and sulfur differ in their. Bonds between dissimilar atoms in molecules inevitably have dipole moments though they are sometimes.
Why Sulfur dioxide is a polar. Here we will discuss about facts whether so2 polar or nonpolar. This means that there is one side top or bottom of the molecule that has both oxygen atoms on it which gives it a slightly negative charge.
If you look at the Lewis structure for SO2 we can see that it is not a symmetrical molecule. SeO2 however is polar with a 120-degree O-Se-O bond angle you need only apply. Whereas SO2 molecule is bent shaped and is polar because of the electronegativity.
While the left and right sides are the same there is a lone pai.

Best Overview Is So2 Polar Or Nonpolar Science Education And Tutorials
Vitamin E Succinate C33h54o5 Pubchem

Is So2 Polar Or Nonpolar Techiescientist

Is So2 Polar Or Nonpolar Science Trends
So2 Polar Or Nonpolar What S Insight

Is So2 Polar Or Nonpolar Science Trends

Density Functional Theory And Thermodynamics Modeling Of Inner Sphere Oxyanion Adsorption On The Hydroxylated A Al2o3 001 Surface Langmuir

Is So2 Polar Or Nonpolar Quora

Pdf Solucionario De Quimica 10 Ed Raymond Chang Alejandra Zamudio Academia Edu

Is So2 Polar Or Nonpolar Polarity Of Sulfur Dioxide
Is So2 Polar Or Nonpolar Quora

Organic Chemistry 1 Flashcards Chegg Com

Chemistry Revision Notes 2012 Pdf
Is So2 Polar Or Nonpolar Polarity Of Sulfur Dioxide
Best Overview Is So2 Polar Or Nonpolar Science Education And Tutorials

Why Can The Polar Gases Like Nh3 So2 And Hcl Be Easily Liquified Quora

Makethebrainhappy Is So2 Polar Or Nonpolar